I-SARS-CoV-2
-
Isixhobo senkqubo eqinileyo ye-SARS-cov-2 antign
Bhala I-500210 Ukucaciswa 1 Uvavanyo / ibhokisi Umgaqo-siseko wokufumana I-Immunocromatographic Assay IMIHLA AmatheUkusetyenziswa kwenjongo AmandlaIsixhobo senkqubo yenkqubo ye-SARS-2ntig-2Antign isebenzisa u-Ignocgrographromagraphromagraphroomatographrographromatographromatographromapographrographromatographroomatographroomatographroomatographroomatographroomatographroomatographroomatographroomatographroomatographrographroomatographroomatographroomatographroomatographrographromatographromatographroomatographroomatographrographroomatographroomatographroomatographroomatographroomatographroomatographroomatographrographromatographromatographromatographroomatographroomatographroomatographroomatographroomatographroomatografi eyokufumana i-SARS-COV-2 nucleocapsid antign kwithenda yabantu. Olu vavanyo lusetyenziswa kuphela kwaye lwenzelwe ukuzivavanya. Kucetyiswa ukuba usebenzise olu vavanyo kwiintsuku ezisi-7 zophawu lwangaphakathi. I-LT ixhaswe luvavanyo lwentsebenzo yedayisi. -

I-SARS-cov-2 antign yovavanyo olukhawulezayo (i-nasal)
Bhala 500200 Ukucaciswa Iimvavanyo ezi-1; iimvavanyo ezi-5 / ibhokisi engama-20; iimvavanyo ezingama-20 Umgaqo-siseko wokufumana I-Immunocromatographic Assay IMIHLA I-Anterior Bal Swab Ukusetyenziswa kwenjongo StrongStep® SARS-CoV-2 Antigen Rapid Test Cassette employs immunochromatography technology to detect the SARS- CoV-2 nucleocapsid antigen in human anterior nasal swab specimen. Olu ncedo lungahambi kakuhle kwaye lwenzelwe ukuzivavanya. Iyahlanjululwa ukuze isebenzise olu vavanyo kwiintsuku ezi-5 zophawu lwe-Out. Ixhaswa luvavanyo lwentsebenzo yeklinikhi. -

I-SARS-cov-2 antign yovavanyo (ukusetyenziswa ngobungcali)
Bhala 500200 Ukucaciswa Iimvavanyo / ibhokisi Umgaqo-siseko wokufumana I-Immunocromatographic Assay IMIHLA I-Anterior Bal Swab Ukusetyenziswa kwenjongo StrongStep® SARS-CoV-2 Antigen Rapid Test Cassette employs immunochromatography technology to detect the SARS- CoV-2 nucleocapsid antigen in human anterior nasal swab specimen. Olu ncedo lungahambi kakuhle kwaye lwenzelwe ukuzivavanya. Iyahlanjululwa ukuze isebenzise olu vavanyo kwiintsuku ezi-5 zophawu lwe-Out. Ixhaswa luvavanyo lwentsebenzo yeklinikhi. -
1人份抗原卡实物图唾液版1_00_副本-300x216.png)
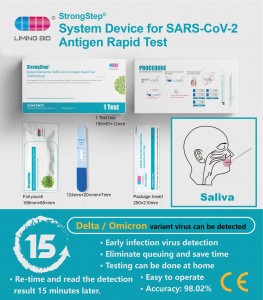
I-SARS-cov-2 i-antign yovavanyo lwe-Antign yamathe
Bhala 500230 Ukucaciswa Iimvavanyo ezi-20 / ibhokisi Umgaqo-siseko wokufumana I-Immunocromatographic Assay IMIHLA AmatheUkusetyenziswa kwenjongo Eli li-cunomomatographic ye-cunomomatographic ye-cunomomatographic yokufumanisa iprotein ye-chacle ye-Antign e-Antigen kwi-Salviva eqokeleleni ngenxa yeentsuku ezintlanu zokuqala kweempawu. I-Assay isetyenziswa njengoncedo ekufunyanisweni kweCovid-19. -

Isixhobo seNkqubo ye-SARS-2 kunye ne-2 kunye ne-Fousea A / B Comfo Antigen Uvavanyo
Bhala 500220 Ukucaciswa Iimvavanyo ezi-20 / ibhokisi Umgaqo-siseko wokufumana I-Immunocromatographic Assay IMIHLA I-Nasal / Oropharyngeal Swab Ukusetyenziswa kwenjongo Eli li-cunomatomagraphic ye-Itromatomatographic ngokukhawuleza ekufumaneni iprotein ye-SARS-2 ye-chacleocapsen kwi-Antign / i-Swabysual Swab eqokelelwe kwiCodd-19 ngomboneleli wezempilo kwiintsuku ezintlanu zokuqala kweempawu. I-Assay isetyenziswa njengoncedo ekufunyanisweni kweCovid-19. -

Isixhobo se-biosaf ye-biosaflity ye-biosaf ye-SARS-cov-2 antign
Bhala I-500210 Ukucaciswa Iimvavanyo ezi-20 / ibhokisi Umgaqo-siseko wokufumana I-Immunocromatographic Assay IMIHLA I-Nasal / Oropharyngeal Swab Ukusetyenziswa kwenjongo Eli li-cunomatomagraphic ye-Itromatomatographic ngokukhawuleza ekufumaneni iprotein ye-SARS-2 ye-chacleocapsen kwi-Antign / i-Swabysual Swab eqokelelwe kwiCodd-19 ngomboneleli wezempilo kwiintsuku ezintlanu zokuqala kweempawu. I-Assay isetyenziswa njengoncedo ekufunyanisweni kweCovid-19. -

I-Novel Coronavirus (i-SARS-CoV-2) I-Interp-Ixesha lokwenyani
Bhala I-500190 Ukucaciswa Iimvavanyo ezingama-96 / ibhokisi Umgaqo-siseko wokufumana I-pcr IMIHLA I-Nasal / Nasopharmal Swab Ukusetyenziswa kwenjongo Oku kwenzelwe ukuba isetyenziselwe ukufumanisa ukufunyanwa okukuko kwe-SARS-2 i-villa ekhutshwe kwi-NasonAlNgabs, i-Oropharyngezwal Swabs, i-Sputum kunye neQonga lokukhutshelwa kwe-FDA / ye-IVD ye-PCR edweliswe apha ngasentla. Ikhithi yenzelwe ukusetyenziswa ngabasebenzi abaqeqeshiweyo
-

I-SARS-cov-2 kunye ne-Feluenza A / B I-Acculx Pay-Times-Ixesha le-PCR
Bhala I-510010 Ukucaciswa Iimvavanyo ezingama-96 / ibhokisi Umgaqo-siseko wokufumana I-pcr IMIHLA I-Nasal / Nasopharyngel Swab / OropharyNgyngel Swab Ukusetyenziswa kwenjongo I-Fortstep® sars-cov-2 kunye ne-Insuelweni A / B I-Indesx ye-PRCR ye-PCRE yenzelwe ukuboniswa kwenqanaba ngaxeshanye kwi-SARS-2, intsholongwane ye-FRUS kunye ne-Swabsngeal Swab okanye i-oropharyngeal swabs kunye ne-chableal ye-wiab okanye i-oropharyngeal swabs (eqokelelwe kwi-Swabs sempilo ehambelana nombosuleleko lwempilo ohambelana nolwazelelo lokuphefumla.
Ikhithi yenzelwe ukusetyenziswa ngabasebenzi abaqeqeshiweyo
-

I-SARS-cov-2 IGM / IGG i-Anti yovavanyo olukhawulezayo
Bhala I-502090 Ukucaciswa Iimvavanyo ezi-20 / ibhokisi Umgaqo-siseko wokufumana I-Immunocromatographic Assay IMIHLA Igazi liphela / i-serum / iplasma Ukusetyenziswa kwenjongo Esi sisiqinisekiso se-chromatographic se-chromatographic se-Igm kunye ne-igg antibodies kwi-SARS-2 virus kwigazi liphela, i-serum okanye iplasma. Uvavanyo lunqunyelwe e-US ukuba lubizwe kwiilebhu eziqinisekiswe yi-CANI ukuze wenze uvavanyo oluphezulu.
Olu vavanyo aluzange luhlolwe yi-FDA.
Iziphumo ezibi aziphikisi ukuba usuleleko lwe-SARS-cov-2.
Iziphumo ezivela kuvavanyo lwe-antidy akufuneki zisetyenziselwe ukufumanisa okanye ukukhupha ngaphandle i-SASE-2 isulelo.
Iziphumo ezifanelekileyo zinokubangelwa kukusuleleka okwedlulileyo okanye ezikhoyo kunye ne-S-TOV-2 coronavirus, ezinjenge-Coronavirus Hku1, i-COROVIRUVUVUVUVIRUS Hku1, NL63, okanye 229e.







